5 reasons to update REACH registrations
The European Chemicals Agency (ECHA) has declared the compliance of REACH registrations as a priority for the year 2019, in particular for potentially hazardous substances. Beyond regulatory compliance, updating records is part of a corporate social responsibility (CSR) approach. Our team of experts on REACH presents 5 reasons to update the dossiers.
In early October 2018, the German Federal Institute for Risk Assessment (BfR) published its study on the compliance of manufacturers with REACH. The study is based on the information available in the REACH registration dossiers for substances with a volume of 100 tonnes or more per year. The REACH compliance study carried out by the BfR and the German Environment Agency (UBA) leads to the same conclusion as ECHA in its work to assess registration dossiers: in the majority of open dossiers, additional information on chemical safety should be requested. The results showed that one to two thirds of REACH registrations in the highest tonnage bands violate the information requirements of REACH. This is similar to observations collected by ECHA during its compliance checks.
ECHA has published its annual assessment report, providing updated statistics on assessed substances and registration dossiers, and advising registrants on how to improve the information provided on chemicals. In 2018, ECHA verified the compliance of 286 registrations, most of which were for substances of potential concern. ECHA verifies that the information is essential the substance is carcinogenic, mutagenic and reprotoxic; or persistent, bioaccumulative and toxic (PBT, vPvB). In 211 cases, the Agency requested additional information from the registrants indicating that the substance was used in a safe manner. ECHA also examined 198 testing proposals. In total, the Agency adopted 274 final decisions, in which 888 different information requests were requested. Positive point: at the request of ECHA, most registrants update their registrations with compliant information.

Based on this observation, ECHA communicates with companies, associations and professional federations to encourage updating and improve the quality of files.
1. UPDATE BECAUSE REACH REGULATIONS REQUIRE IT
The REACH regulation requires a spontaneous update by the registrant in the following cases (article 22):
- change in its status (as a manufacturer, importer or producer of articles, for example) or its identity (name or address, for example);
- change in the composition of the substance;
- modification of the annual or total quantities manufactured or imported or the quantities of substances present in articles produced or imported if this results in a change in the tonnage band, including the cessation of manufacture or import;
- new uses identified and / or advised against;
- new knowledge concerning the risks presented by the substance for human health and / or the environment of which the registrant may reasonably have become aware and which leads to modifications in the safety data sheet or in the chemical safety report;
- modification of the classification and labeling of the substance;
- update or modification of the chemical safety report (CSR) or of annex VI, section 5 “Guidance on Safe Use” (GSU) (note that the update of the CSR may have an impact on the extended SDS (FDSe) whose exposure scenarios (ES) derive from the risk assessment (CSA) of the REACH registration dossier);
- testing proposal decision, a process aimed at limiting experimentation on vertebrate animals;
- modification concerning the access granted to the recording information (confidentiality requests, etc.).
Most of these data are detailed in Annex VI of REACH and called "individual" because they are specific to the substances and to the activity of each company (legal entity) co-declaring a registration. They are key parameters to take into account for the risk assessment (CSR), which is why it is essential to keep them up to date. They are submitted both by the lead registrant (LR) and by the members of the joint submission.
Currently, the REACH regulation requires companies to update their registrations with new relevant information "without undue delay". ECHA emphasizes the need to better define the deadline, and what is meant by excessive delay in order to take adequate sanctions when necessary. The communication from ECHA of 21 February 2019 suggests that more binding rules for updates are expected, in particular through the forthcoming publication of an "implementing act" amendment to REACH.
2. UPDATE IN PLANNING OF AN ASSESSMENT OF THE COMPLIANCE OF THE FILE BY THE AUTHORITIES
ECHA can examine any registration dossier to verify that the information submitted by registrants complies with legal obligations. The compliance checks (target "or comprehensive") assess the description of the identity of the substance, the safety information contained in the dossier, including the chemical safety report (CSR), or parts dossier specific - for example, information concerning the protection of human health. Following these compliance checks, ECHA may request additional information from the registrant (s) within a certain time limit. It can also change the identity of substances by proposing a "substance identity change" (SID) and potentially modify the EC and CAS numbers.
REACH is based on the principle that registrants must ensure that substances used and placed on the market do not harm human health or the environment. Therefore, registrants are legally required to submit a registration dossier that complies with REACH information requirements and to keep their dossiers up to date with the latest information to be valid on the market.
When evaluating the dossier, ECHA only takes into account the dossier updates submitted before issuing a draft decision. If new information becomes available subsequently, registrants should submit it through their comments to the draft decision. The Agency will take into account the information contained in the comments and may modify the decision accordingly, which is why it is important to respond to them. After this 30 day period, registrants cannot retrospectively downgrade their tonnage band or withdraw certain uses from their registration to remove information requests from ECHA decisions. Indeed, the registration dossier must be complete with regard to the REACH information requirements for the tonnage band registered and the uses at the time of the opening of the dossier evaluation.
3. UPDATE BECAUSE THE WRITING OF FILES CHANGES
Since its publication, the implementation of the REACH regulation and the drafting of registration dossiers have evolved considerably, both in terms of requirements and tools.
Indeed, ten technical guides have been published with the aim of facilitating the implementation of REACH by describing good practices related to compliance with obligations.
In addition, the IUCLID (version 6) and CHESAR (version 3) software have been developed and improved over time in order to optimize the drafting of registration dossiers and health risk assessment reports and the environment (CSR).
Finally, the reinforcement of the technical compliance check "Technical Completeness check" (TCC) controls since June 21, 2016 the files during the submission, by the implementation of automated rules and additional manual checks carried out by ECHA.
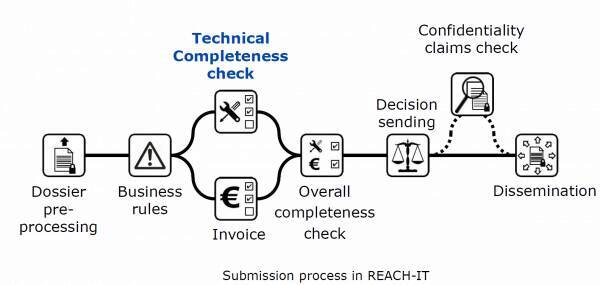
In some cases, the submission is rejected and a "Technical completeness check (TCC) letter" is issued to the registrant who will have a second attempt to submit his REACH registration. A second "Failure of technical completeness check" (TCC) rejection will result in a rejection of the submission and a loss of the fee paid to ECHA, hence the importance of taking into account the latest technical recommendations.
4. UPDATE BECAUSE YOUR SUBSTANCE IS A NANOMATERIAL
An Implementing Regulation amending the Annexes of REACH was published in the Official Journal of the EU on Tuesday 4 December. The objective of this amendment is to better take into account nanomaterials, and will be applicable from 1 January 2020: the REACH registration dossiers for existing nanosubstances must therefore be updated by incorporating the specificities of nanomaterials by the end. of the year 2019. Upcoming recordings must therefore:
- contain information on the basic characteristics and use of nanosubstances
- assess how to handle them safely,
- assess the risks they represent for health and the environment and how these health and environmental risks can be adequately controlled.
The new provisions will have to be applied to all nanoforms of substances falling within the scope of REACH, from “traditional” nanomaterials already widely used and registered to nanomaterials specifically designed and placed on the market by companies working on these innovations, often of TPE / SME size.
5. UPDATE BECAUSE THE PROCESS IS FREE
With the exception of certain updates (change of legal entity, increase in the tonnage band) referred to in Regulation 2015/864 on fees and charges due to ECHA in application of REACH, the updates do not are not subject to a fee for the European Agency.
In the case of registration dossiers initially submitted in 2010 and 2013, the work of drafting and consolidating the dossiers involved in the update can, however, be significant and costly. It can also be an opportunity to bridge the gap between the terms of the sharing of costs and data (“SIEF agreement”, letter of access “LoA”) to check whether it is necessary to request a reimbursement of the overpayment. perceived "refunding" to the main registrant (LR), or on the contrary to pool efforts or costs to promote consistency of files.
ATOUT CHIMIE has been building dossiers since the advent of REACH more than 10 years ago now, and has updated various complete registrations submitted during the 2010 and 2013 deadlines in particular. From our experience, the completeness of the files initially submitted greatly influences the updating work necessary to achieve current quality standards.
Also, our team of (eco) -toxicologists offers you a quality audit of your files. We also support manufacturers during inspections (DREAL), assessment of files (ECHA, Member States), and in responding to public consultations that may lead to harmonized classifications, restrictions or even authorizations (SVHC). Contact us at: info@atoutchimie.eu
